Vaccine
Talk
(Egyptian Edition)
"Everything you need to know about
vaccines in Egypt"
(Egyptian Edition)
"Everything you need to know about
vaccines in Egypt"
Herpes zoster (shingles) is caused by reactivation of varicella‑zoster virus (VZV), the virus that causes chickenpox.
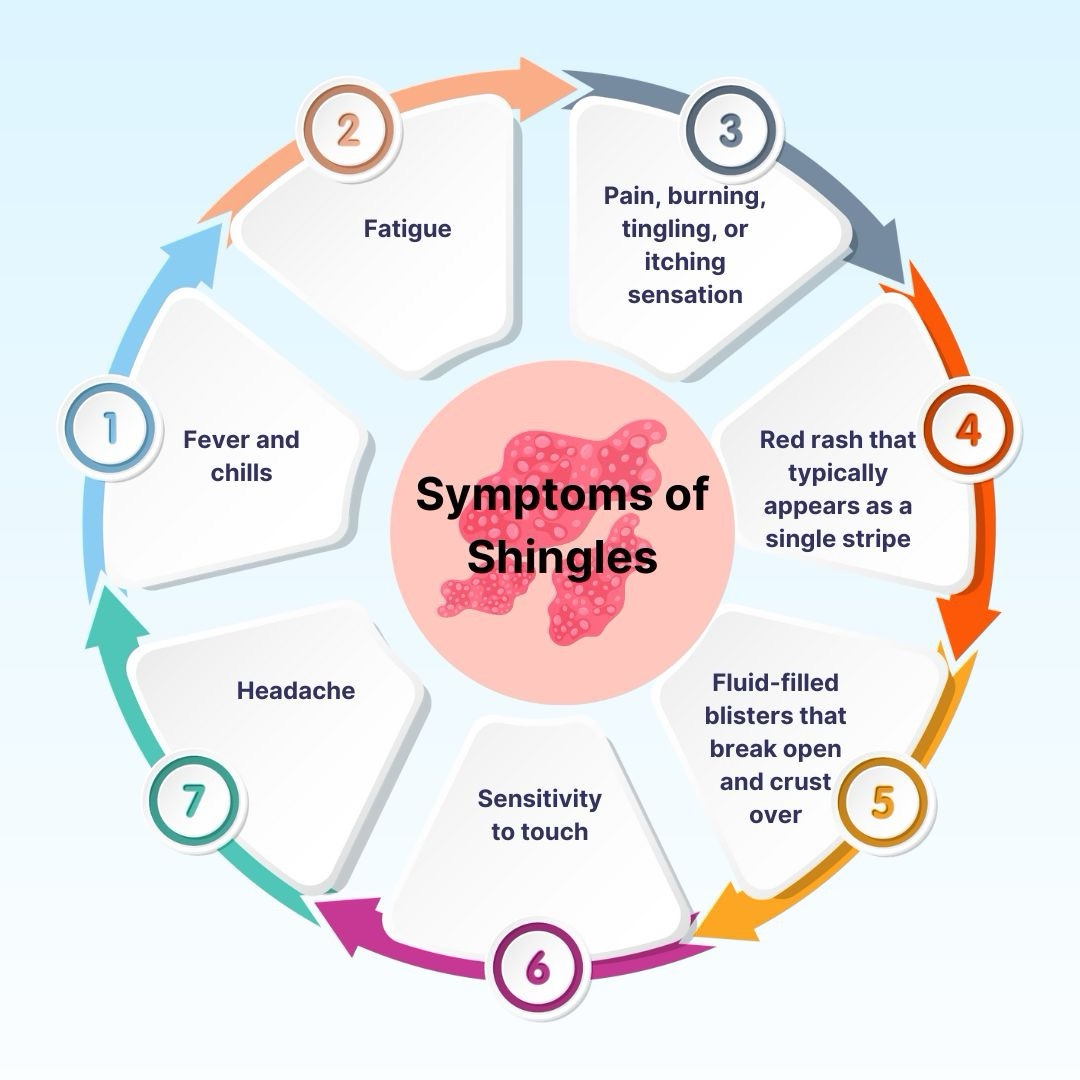
After primary infection, VZV remains latent in dorsal root/cranial nerve ganglia and can later reactivate—more likely with immune senescence or immunosuppression—producing a painful dermatomal rash.
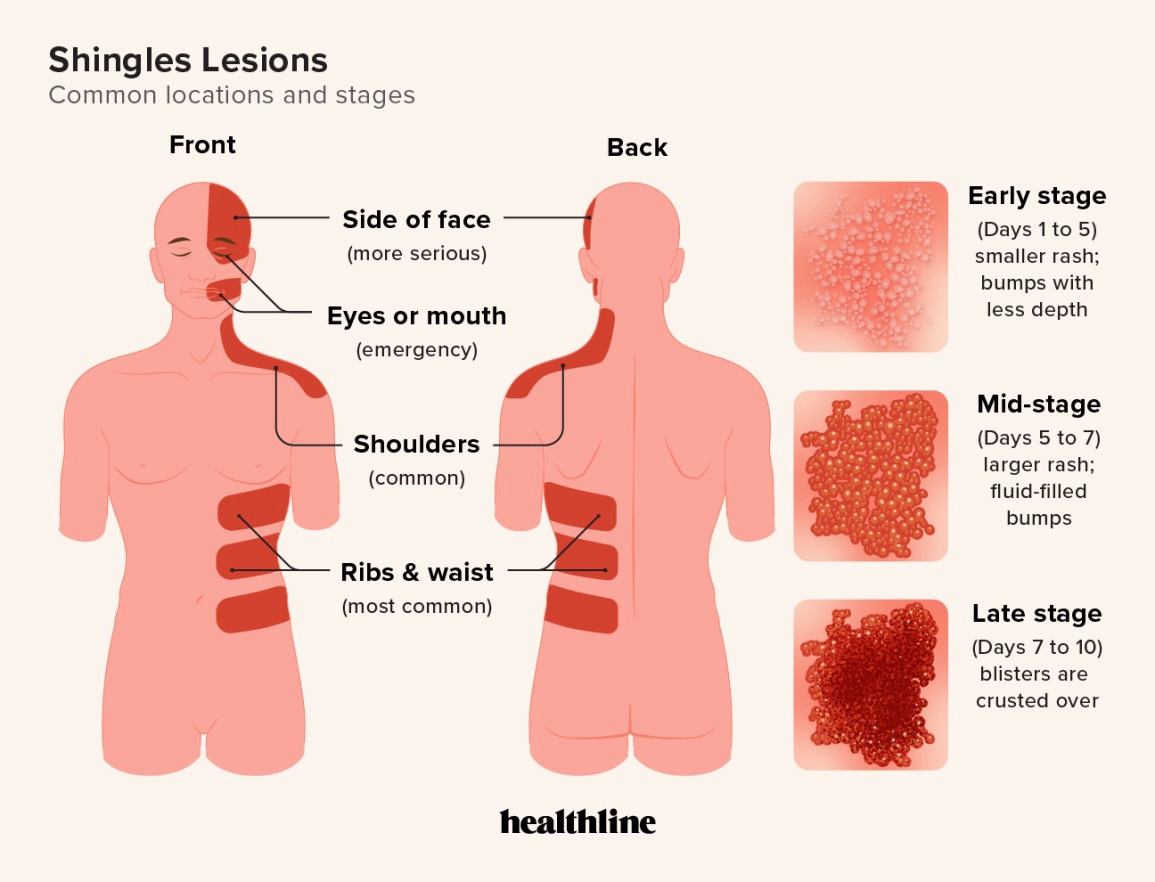
The rash is typically a unilateral stripe of vesicles in one or more dermatomes; facial/ophthalmic involvement requires urgent evaluation.
Shingles itself is not transmitted person‑to‑person; however, fluid from shingles vesicles can transmit VZV to a susceptible person (causing chickenpox, not shingles). Vaccination is highly effective at preventing shingles and its complications. Early antivirals reduce severity and duration.
Shingles cannot be directly "caught." VZV from vesicle fluid can infect a non‑immune person and cause chickenpox. Risk falls once lesions crust.
Keep rash covered; avoid touching; frequent handwashing
Avoid contact with susceptible pregnant women, newborns, and immunocompromised persons
Clinical—unilateral dermatomal vesicular eruption. If uncertain/complicated, test vesicle material for VZV (PCR).
WHO recommends recombinant zoster vaccine in a two‑dose schedule (≥2‑month interval) for adults ≥50 years and those with chronic conditions in settings where herpes zoster is a significant public‑health issue. The vaccine reduces risk of shingles and PHN and is indicated even after a prior episode.
In Egypt, the Egyptian Drug Authority approved Shingrix on 11 Sep 2023; public launch occurred 26 Apr 2024 (GSK event). Varicella vaccination in childhood lowers lifetime shingles risk versus natural infection.
References:CDC – Shingles |WHO – Herpes Zoster |EDA – Shingrix PI (2023)
المصدر: الهيئة المصرية للدواء / وزارة الصحة
لا توجد علاقة مباشرة بين الموقع وهذه الشركات، والمحتوى لأغراض التوعية فقط. ولا يجوز استخدامها في أي أغراض تجارية